A Randomised Controlled Trial of Sildenafil Therapy In Dismal Prognosis Early-Onset Intrauterine Growth Restriction
Early-onset placental intrauterine growth restriction (EO IUGR) is associated with a high risk of perinatal morbidity and mortality. In association with reduced circulating placental growth factor (PLGF) severe EO IUGR results from abnormal placentation with inadequate remodelling of the maternal uteroplacental arteries. There is no known treatment for placental IUGR. Management involves intensive fetal surveillance with delivery with evidence of serious fetal compromise. However, remote from term, delivery is associated with significant perinatal mortality and morbidity.
STRIDER Canada is one of a consortium of STRIDER randomised controlled trials (RCTs) each of which will be designed to determine whether or not maternal treatment with sildenafil citrate improves markers of perinatal wellbeing.
Aim
To determine whether maternal treatment with oral sildenafil citrate improves perinatal outcomes in pregnancies complicated by severe early-onset IUGR without increasing risks to the mother.
Trial design
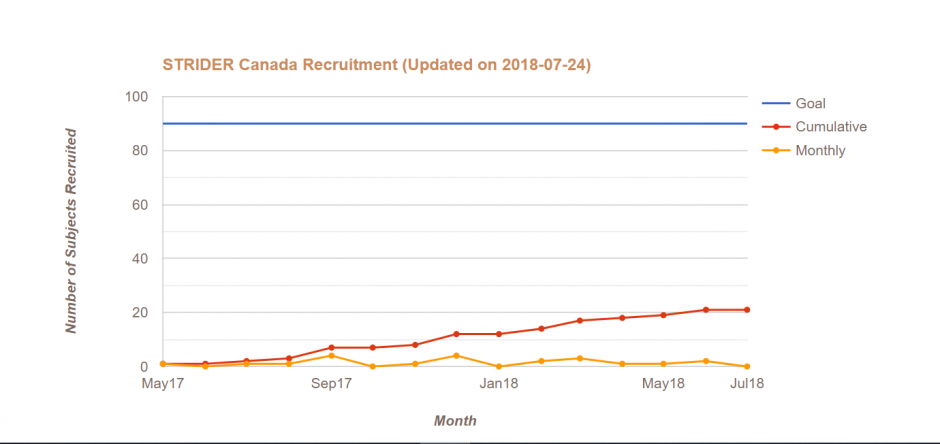
STRIDER Canada is designed as investigator-initiated double-blind, randomised placebo-controlled trial of 90 women with a diagnosis of early-onset intrauterine growth restriction with an intention-to-treat analysis.
Study population: All legally adult women with a diagnosis of a pregnancy affected by early-onset IUGR between 18+0 and 27+6 weeks of gestation, with a singleton pregnancy and serum PLGF <5th percentile for gestational age will be considered for randomisation.
Intervention: Sildenafil 25mg or placebo tablet orally three times daily.
Duration of treatment and participation: The first dose will be administered shortly after randomisation, between 18+0 and 27+6 weeks. The last treatment will be given at delivery or 31+6 weeks, whichever is sooner.
Primary outcome: The primary outcome will compare the gestational age at delivery (d) between sildenafil- and placebo-treated groups.
Randomisation System
STRIDER Canada uses iSTAR (integrated System for Trial Allocation and Randomisation), which is developed by UBC PRE-EMPT, for randomisation and drug dispensing. For more details about iSTAR, please click here.
Updates
The STRIDER Canada trial has been stopped. The trial team is currently analyzing the data.
Principal Investigators
- Peter von Dadelszen and Kenneth Lim
Co-investigators
- Laura Magee and Sayrin Lalji
Perinatal Clinical Trials Research Manager
- Chirag Kariya
Funder
- Canadian Institutes of Health Research (operating grants)